
AdvaMedDx
AdvaMedDx promotes innovation and expanded access to quality testing.
AdvaMedDx and the Value of Diagnostics

AdvaMedDx, a division of the Advanced Medical Technology Association (AdvaMed), represents over 70 manufacturers of innovative in vitro diagnostic (IVD) tests in the U.S. and abroad. AdvaMedDx seeks to advance policy to promote innovation and expand access to quality testing.
Across the health care delivery system, diagnostic tests are critical for the day-to-day evidence-based care of all patients by enabling early detection of disease, aiding in the determination of the most appropriate clinical pathway for patient care, and increasingly supporting the practice of precision or personalized medicine to improve the health and health care of all patients.

AdvaMedDx Members
Our members represent the world’s leading diagnostics manufacturers alongside the next generation of companies developing innovative diagnostic tests and technologies in the U.S. and abroad.
Diagnostics Regulatory Reform
AdvaMedDx supports the modernization of the regulatory framework for all clinical diagnostic tests to foster innovation, embrace scientific advances, and ensure swift access to accurate and reliable tests for all patients.

Coverage & Payment
Diagnostic Tests Coding, Coverage, and Payment
Diagnostic tests provide vital information in the management and care of patients. Appropriate coding, coverage, and payment policies for diagnostic tests are crucial to ensuring these products and services are accessible to the patients who need them.
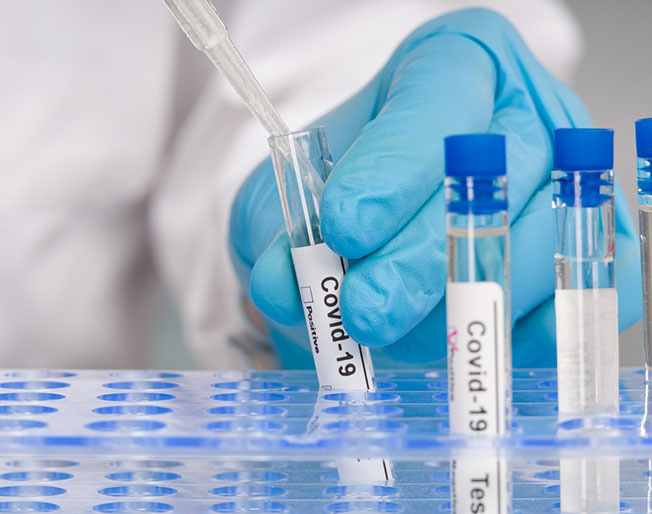
COVID-19
Diagnostics & COVID-19
Diagnostics manufacturers are delivering millions of COVID-19 tests across the U.S. and globally each week. Testing is an essential tool to our pandemic response and recovery.
AdvaMedDx Partners
AdvaMedDx collaborates with diagnostics associations across the country and around the world.
Become a Member
As an AdvaMedDx member, you have the opportunity to shape the future of the industry with like-minded peers.